A long-awaited clinical trial of a drug aimed at improving daily function in the early stages of Huntington’s disease has produced negative results, Prilenia Therapeutics announced on April 25 at the American Academy of Neurology meeting in Boston.
According to Prilenia, the drug, pridopidine, failed to show improvement for trial participants on its primary and secondary measurements of symptoms (endpoints).
"Unfortunately, the failure of the PROOF-HD trial to meet its primary endpoint in preliminary analyses is a huge disappointment for the HD community,” Jody Corey-Bloom, M.D., Ph.D., the director of the Huntington’s Disease Society America (HDSA) Center of Excellence in San Diego, wrote me in an e-mail on April 25. “There was, however, some suggestion of benefit on other clinical measures, particularly a computerized assessment of motor function [a person’s movements], and I suspect we will see additional detailed analyses in the coming months.”
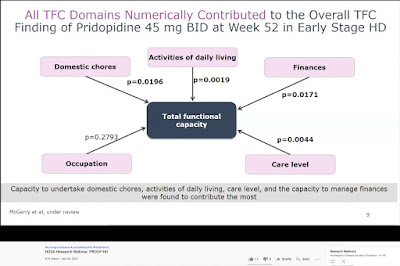
“We won’t sugar-coat this: the trial results were unfortunately negative,” the HD science site HDBuzz concluded. “The drug did not slow progression of HD as measured by the TFC [Total Functional Capacity]. Failing to meet its primary endpoint means that pridopidine will not get licensed by the FDA and other regulatory agencies.”
“The data obtained as part of the study remain useful and will be analyzed further for new insights they can provide,” Martha Nance, M.D., the HDSA Center of Excellence director in Minneapolis, wrote me. “We will not stop looking for better answers.”
Seeking a disease-modifying therapy
As an HD gene carrier who will inevitably develop symptoms, I had hoped to take pridopidine – if prescribed by a doctor – to help prevent or slow disease onset and maintain my daily activities. At 63, I have reached far beyond my HD-stricken mother’s onset in her late 40s. She died at 68.
Pridopidine would have been the first drug to potentially affect the progression of HD. Also, unlike drugs in other key trials that require spinal injection or direct injection into the brain (requiring an operation), pridopidine is a pill.
The news about pridopidine is the latest in a series of disappointing clinical trial news over the past two years. Last October, the highly innovative Triplet Therapeutics, Inc., had to shut down because of investor nervousness about the HD field. In December, Novartis had to end a program of developing its own pill for HD, branaplam, because of serious side effects in volunteers in a clinical trial.
PROOF-HD was a definitive Phase 3 trial. There are no other Phase 3 HD trials in progress for disease-modifying therapies.
Researchers remain hopeful that they will ultimately develop effective therapies. In January, Roche announced the start of GENERATION HD2, a second, less ambitious clinical trial of its gene-silencing drug tominersen. Other trials are in progress. This week, scientists and drug hunters focused on the cutting-edge developments at the 18th Annual HD Therapeutics Conference in Dubrovnik, Croatia (click here to read more).
Prilenia CEO and founder Michael Hayden, M.D., Ph.D., reporting the negative results for improvement in Total Functional Capacity in PROOF-HD participants at the HD Therapeutics Conference, April 27, 2023 (photo from HDBuzz)
Stepping back from advocacy
With the rest of the HD community, I have been riding the “clinical trial roller coaster” of emotional ups and downs in the quest for therapies.
I am also acutely aware of the passage of time – and that I may never get to participate in a clinical trial. GENERATION HD2, for example, restricts participation to ages 25-50. In the 30 years since the discovery of the huntingtin gene, we still have not seen a therapy to slow, stop, or reverse the disease. This month marks 25 years of my work as an HDSA volunteer advocate.
Recently, due to unforeseen circumstances unrelated to HD, I have had to step back from my advocacy, including this blog. My current challenges come on top of the juggling act that has characterized my fight against HD since my mother’s diagnosis in 1995 and positive test for the mutation in 1999.
So I have struggled to maintain my sense of meaning and purpose – crucial in slowing cognitive decline in HD.
I have thus felt the need to concentrate on self-care. I believe there is wisdom in recognizing the importance of self-care – especially for someone like me facing a challenge for which science has yet to find an answer.
As I have learned, my first obligation is to my own health – remaining symptom-free and therefore fully available for my family, friends, and others I love and care for.
In this blog I have aimed for transparency about my health. For my own and the community’s benefit, I have sought to report accurately and thoughtfully on the quest for therapies, the endeavor to provide better care, and the many ramifications of HD (click here to read more).
Prioritizing diet, sleep, and exercise
So, as an advocate striving to be the best version of himself, I want to embrace self-care.
Last August and September, though fully vaccinated and having masked except for meals, I fought off an infection of COVID-19 caught in Boston while attending HD2022: Milton Wexler Biennial Symposium, sponsored by the HD-focused Hereditary Disease Foundation. I have been deeply concerned about COVID-19 because of the many instances of long COVID, the disease’s neurological symptoms, and the devastating impact on people in their 60s and older.
The pandemic has highlighted the need for self-care and caregiving.
To continue HD-free, I must prioritize the triad of a healthy diet, adequate sleep, and regular, vigorous exercise. I tell myself about swimming: in terms of health, “this is the most important thing you can do today.”
Recently, after challenges replacing my retired psychotherapist of 24 years, I began working with a new therapist – a profound form of self-care.
Self-care for me also includes continued participation in Enroll-HD and other HD research studies.
Gene Veritas (aka Kenneth P. Serbin) swimming in his backyard pool (family photo)
Addressing deficits in care and caregiving
With the negative results from PROOF-HD and other recent trials, I am reminded of the debate and tensions over “care versus cure” that I witnessed at the start of my HDSA advocacy in the late 1990s and early 2000s. We need both. I saw my mother’s desperate need for care as HD devastated her and fought with fellow advocates to support the search for a cure.
As I age, and with the continued lack of effective therapies, the likelihood that I will not take a disease-modifying drug increases – as does, therefore, the likelihood that I will need caregivers. For HD gene carriers like me, improvements in caregiving will become essential.
As one key study has shown, serious deficits HD in care and caregiving remain, and plans for addressing this issue remain aspirational.
Most HD funding goes to biomedical research. Greater resources for HDSA and other patient advocacy organizations could help address the care and caregiving shortfalls.
Being patient and gentle with human fragility
Upon entering this world, we are all subject to illness, both genetic and non-genetic. The fight against HD, COVID-19, and other illnesses highlights our human fragility.
We are all potential caregivers and recipients of care, at various times in our lives.
I have found heart in the words of Pope Francis during his historic May 2017 audience with the HD community at the Vatican, which I attended with my family: “Fragility is not an ill, and disease, which is an expression of fragility, cannot and must not make us forget that in the eyes of God we are priceless. Disease can also be an opportunity for encounter, for sharing, for solidarity.”
As I patiently and gently await the recharging of my mental and spiritual batteries, I am mindful of my own – and others’ – fragility and the new opportunities for solidarity in achieving research progress and improved caregiving.
.jpeg)