This article is in commemoration of Huntington’s Disease Awareness Month (May).
One of the most impactful university labs focusing on Huntington’s disease, the X. William Yang Research Group at the University of California, Los Angeles (UCLA) employs a multi-pronged approach to investigating potential therapies for this deadly brain disorder.
Started in 2002 by X. William Yang, M.D., Ph.D., the lab has produced several key findings on HD, mainly through the study of genetically modified (i.e., transgenic) mice, engineered to carry the HD mutation and exhibit some of the disease-like phenotypes (characteristics).
Dr. Yang was inspired to focus on Huntington's disease because of his interaction with patients in Venezuela – the world’s largest clusters of HD families – and the HD scientists working there. In 2000 and 2002 he was invited to observe these families and assist with studies by Nancy Wexler, Ph.D., the president of the HD-centered Hereditary Disease Foundation (HDF) and leader of the landmark effort to identify the HD gene in 1993.
Dr. Yang's Venezuela experience cemented his resolve to study HD in his own lab. Indeed, the first research grant ever received by Dr. Yang was from HDF. Today he serves as its scientific advisory board’s vice chair.
Dr. Yang’s team has also collaborated with CHDI Foundation, Inc., the largest private funder of HD therapeutic research. Pharmaceutical firms such as Roche (the world’s largest) and Ionis Pharmaceuticals, Inc., the developer of the Roche drug now in its second HD clinical trial, have consulted Dr. Yang for his expertise.
Dr. Yang has emerged as a leading academic voice in HD science. Listed as the first author, in February he and two other important prominent HD researchers – Leslie Thompson, PhD., of UC Irvine and Myriam Heiman, Ph.D., of the Massachusetts Institute of Technology (MIT) – published a major co-edited book. Huntington’s Disease: Pathogenic Mechanisms and Implications for Therapeutics presents the latest work on the disease’s medical impact, genetics, the huntingtin protein, new tools and models for research, and an overview of therapeutic approaches and clinical trial programs.
The back and front covers of Huntington’s Disease: Pathogenic Mechanisms and Implications for
Therapeutics (image courtesy of Dr. Yang) (Click on an image to enlarge it.)
‘The stars are aligned’ for developing HD treatments
Although the use of human data in HD research has increased dramatically, crucial research in mice has become more relevant to potential therapies because of new biotechnologies and the availability of so-called “big data” made possible by powerful computing systems.
“This is completely unprecedented in terms of the kind of study we can do,” Dr. Yang told me in a 40-minute interview on January 29, noting the advantages of a “21st century toolbox.” “Mouse models in this context are extremely useful.”
We met in Dr. Yang’s office in his lab, which is located in UCLA’s Gonda (Goldschmied) Neuroscience and Genetics Research Center. I was invited to Los Angeles to offer my perspective as an HD gene carrier on the first day of a two-day HDF scientific workshop, co-chaired by Dr. Yang.
“I know it's probably an oxymoron to say that it's time to be hopeful, because we’ve been to a hopeful stage many times before,” Dr. Yang said, acknowledging the negative results of some recent clinical trials. He added that “the stars seem to be aligned” for developing HD treatments.
Focusing on the brain
Dr. Yang grew up in Tianjin, China, a port city located 80 miles from the capital, Beijing. In 1985, Dr. Yang was one of five students selected by the Chinese government to participate in the Rickover Science Institute, founded by Admiral Hyman G. Rickover to foster high-school science education for both domestic and international students. Rickover developed the first nuclear-powered engines and first atomic-powered submarine.
“I did a whole summer of research at the NIH [National Institutes of Health], working on signaling pathways in rat brains,” Dr. Yang wrote in a follow-up e-mail to our interview. “The research experience got me really interested in studying the mammalian brain.”
The Rickover program is now called the Research Science Institute (RSI). Among other prestigious alumni are Harvard University’s Steve McCarroll, Ph.D., a leading molecular geneticist who also works on HD; and MIT/Broad Institute's Feng Zhang, Ph.D., a CRISPR research pioneer.
After RSI, Dr. Yang briefly studied at Peking University, one of China's top universities, before transferring to Yale University, where in 1991 he completed the highly demanding joint B.S./M.S. program in molecular biophysics and biochemistry.
Over lunch Dr. Yang and I reminisced about our years at Yale. I was privileged to graduate from Yale in 1982. I told Dr. Yang that I had seen Admiral Rickover give a public lecture at the university – a poignant moment for me as a history major because of his military and scientific prominence. I told Dr. Yang of my interest in tracking the contributions of Yale and its graduates like him to HD science and medicine (click here, here, and here to read more.)
Dr. Yang completed the joint M.D./Ph.D. program at The Rockefeller University (Ph.D., 1998) and Weill Medical College of Cornell University (M.D., 2000) in New York City. In 2002, he finished postdoctoral research in the Rockefeller lab of Nathanael Heintz, Ph.D., which focuses on HD and other neurological and psychiatric disorders.
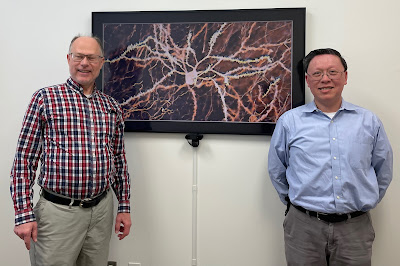
Gene Veritas (left) (aka Kenneth P. Serbin) with Dr. William Yang in his UCLA office. In the background: a mouse medium spiny neuron. In humans this neuron is one of the cells most affected by Huntington’s disease (photo by Nan Wang, Ph.D., of the Yang Research Group).
A ‘trustworthy and versatile’ invention
Dr. Yang and his lab have made key contributions to HD science, including understanding the causes and potential pathways to therapies. The team also studies Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative disorders.
As a Ph.D. student, Dr. Yang co-invented with Dr. Heintz and Peter Model, Ph.D., the first method to engineer Bacterial Artificial Chromosomes (BACs) to generate transgenic mice. BACs have the advantage of holding long strands of DNA with key regulatory elements that confer accurate gene expression in transgenic animals.
In an analysis of this research, which Drs. Yang, Model, and Heintz published in 1997, one leading biologist described their technique as “trustworthy and versatile” for cloning genes and the key task of learning the specific function of particular genes.
Indeed, scientists have used this method to generate a variety of transgenic animals, from zebrafish to mammals (click here to read more).
The key BACHD mouse
In 2008, Dr. Yang and other researchers published the results of a project creating the first BAC transgenic mouse model of HD, the BACHD mouse, their term for this mouse specifically engineered to study HD.
As Dr. Yang explained in our interview, the team inserted a long strand of a mutant (irregularly expanded) human huntingtin gene into the mice. Those genetic characteristics do not normally exist in mice. As they hoped, the mice developed dysfunction, displaying impaired movements, shrinkage to the same brain regions affected in HD, and damage to the synapses (the connections between brain cells).
“We developed different versions of these mouse models to allow us to ask, for example, which cell types in the brain with mutant huntingtin are important,” Dr. Yang said.
The team demonstrated the presence of mutant huntingtin in two key areas of the brain: medium spiny neurons in the striatum and pyramidal neurons (brain cells) in the cortex. (See the photo above with Dr. Yang, me, and an image of a medium spiny neuron. Also see the photo in the next section.)
In mice, humans, and other mammals, the cortex handles important processes such as cognition, memory, motor control, and sensory processing. The striatum – an area deep in the brain and greatly affected in HD – controls motor (movement), motor and reward learning, and executive function. In humans, this region is also known as the caudate and putamen. The Yang lab also examines communication between these regions.
Dr. Yang (left) and Nan Wang, Ph.D., a project scientist focusing on Huntington’s, in the lab (photo by Gene Veritas)
Using mice and genetics to understand HD
In detecting the impact of mutant huntingtin in those areas, that initial BACHD research revealed disease phenotypes in both striatum and cortex, Dr. Yang recalled. “That study turned out to be really important because, for the longest time, people thought the striatum, the medium spiny neuron, was really the primary site of action.”
Removing the mutant huntingtin from the cortex led to improvement in the mice’s behavior and even partially helped the striatum, Dr. Yang explained. Likewise, deleting mutant huntingtin from the striatum brought some improvement.
“But most importantly, if you reduce mutant huntingtin in both cortex and striatum, the BACHD model looks really, really good, almost as good as a normal mouse,” he added.
The BACHD work, he recalled, helped to convince the field that the cortex is one of the key brain regions that should be targeted in HD clinical trials. The Roche/Ionis ASO lowers the level of huntingtin protein more in the cortex than in the caudate/putamen, according to preclinical studies in non-human primates.
In sum, Dr. Yang said, the studies of BACHD mice represent a “proof of concept that we can use this kind of a sophisticated – genetically as accurate as we could get – type of mouse model to inform about disease pathogenesis” – how HD develops, progresses, and, significantly, might be treated.
A mouse medium spiny neuron (image courtesy of Dr. Yang)
From disease switch to vulnerable neurons
The Yang Research Group has achieved other key findings, some in collaboration with other labs.
The Yang lab teamed with researchers at UC Irvine, UC San Francisco, the University of Pittsburgh, and the University of Tennessee to study the chemical modification of the huntingtin protein itself. This research focused on so-called “chemical tags” that naturally attach to the very beginning of the huntingtin protein, a small region acts like a disease switch.
In one experiment, this research used a BACHD-like mouse to mimic the tagging. That resulted in mice that had “very little disease despite having the HD mutation,” Dr. Yang explained. The results were published in 2009.
In 2015, the Yang Research Group published a separate study showing the genetic switch is necessary to prevent severe disease including neuronal loss and movement deficits, phenotypes reminiscent of those found in HD. These studies showed that the huntingtin protein itself and its chemical tags could be a source of new targets to develop therapies, Dr. Yang said.
From watching mice in ‘log rolling contests’ to unbiased genetic analysis
In 2013, the Federal Government announced the launch of the BRAIN initiative to enhance understanding of the human brain. The Yang lab was one of the first 59 in the country to receive support in the initial round of BRAIN funding. It now has its third grant. It receives support from other government agencies, as well as the HDF and CHDI. The lab’s achievements include developing a new, genetic way to label the complete, intricate shape of single brain cells, which allows the study of their function and dysfunction in diseases such as HD.
With big data and "the 21st century toolbox," the field of HD research has advanced from more traditional ways of observing diseased mice to more nuanced molecular, cellular and systems biology analyses, Dr. Yang explained.
In earlier research, by primarily relying on the behavior and pathology of individual mice, the work resulted in “relatively few readouts” of data, Dr. Yang observed. With that methodology, scientists had mice doing activities such as “spontaneously move” in an open area or on a rotarod, “like the ESPN log rolling contest,” he said. Scientists also routinely measured loss of brain matter.
Now, scientists can do a “big-scale, unbiased molecular studies” by examining tens thousands of datapoints, including analysis of DNA, RNA and proteins, Dr. Yang added.
Clues from gene expression about neuronal vulnerability
Collaborating with CHDI, the Yang lab’s work in this area has involved study of HD’s impact in different areas of the brain, moving beyond the standard understanding that most damage comes in the striatum. The lab has done this research using different types of engineered HD mouse models carrying different lengths of CAG repeats and measured the levels of tens of thousands of RNA transcripts ("RNA-seq," that is, RNA sequencing) in the mouse brains and peripheral tissues.
Published in 2016, the results noted that despite the presence of the mutant HD gene throughout the body, the disruption in gene expression in these HD mice is highly selective to the striatum, the most affected brain region in HD. The severity of the disruption is correlated with the length of CAG repeats in these mice. Moreover, the molecular defects in the striatum appear in young adulthood, worsening with age.
“There's about 100 or so genes that have essential function selective to the striatal neurons that are most affected in Huntington’s disease,” Dr. Yang said. “And somehow the mutant huntingtin knows to go there and make them the sickest, which we thought was a remarkable find – a sense that there's some fundamental mechanism connecting this CAG expansion to selective neuronal vulnerability.”
‘Perturbing’ the mice to understand human modifier genes
Taking advantage of the gene signatures from RNA-seq studies, especially those selectively disrupted in the striatum, the Yang lab embarked on a study using such gene signatures to sensitively detect "modifiers" of the disease. To achieve this, they used these genes to genetically “perturb” the mice, Dr. Yang explained.
“We basically genetically perturb the huntingtin mouse and say, ‘which gene, if we perturb them just right, can make the disease worse – that's one thing that's interesting – but more importantly make them better. And if better, how much better.’”
Continuing this line of work, the lab has continued testing the impact of other genes. These experiments include study of some of the human HD modifier genes – about ten – previously identified by the Genome Wide Association Study (GWAS) from over 9,000 HD-affected individuals and their relatives. The modifiers found by the Genetic Modifiers of Huntington's Disease Consortium can delay or hasten HD onset.
In addition, the Yang lab tested over 100 other candidate modifier genes identified in the prior systems biology work.
The scientists have tested large number of genetic mutants in HD mice to determine whether this makes the disease better or worse, Dr. Yang said. Noting that the results are still unpublished, Dr. Yang said that the team is drilling down on discovering the best gene targets that could help advance therapies to alleviate the disease.
Three potential ways to treat HD
Dr. Yang also discussed his outlook for therapies to slow, prevent, or reverse the course of Huntington’s. As noted, he believes that “the stars seem to be aligned” for the development of treatments.
In exchanging ideas with other HD scientists, he proposed the model of a stool – which needs four legs to remain stable – as a metaphor for the benefit of developing multiple therapies (polypharmacy) that could act synergistically for HD.
“If one drug could work for HD, that will be great. However, for many diseases, like HIV or cardiovascular diseases, multiple drugs together can make the disease more manageable, and patients' lives much better.”
As of now, Dr. Yang said scientists are developing three potential legs of the therapeutic stool. Each leg represents a new angle in understanding HD and how it might be applied to slow or stop the disease.
The first leg: huntingtin lowering
As the first leg of the therapeutic stool, Dr. Yang pointed to so-called huntingin lowering – the reduction of the HD gene (DNA), RNA, or its toxic protein in the brain. Pioneered in patients by the above-mentioned Roche/Ionis clinical trial program, this approach has captured the attention of many academic and biopharma labs.
This Roche/Ionis drug is an antisense oligonucleotide (ASO), a synthetic strand of DNA that degrades the RNA from making the huntingtin protein. Other clinical trial programs aim to alleviate HD with ASOs, or other DNA or RNA targeting therapies. Some of them using small chemicals to reduce human huntingtin.
This approach has received ample coverage in this blog and elsewhere.
The second leg: GWAS/mismatch repair genes
Dr. Yang pointed to potential therapies based on the HD GWAS genes – which include DNA mismatch repair (MMR) genes – as the second leg of the stool.
“Lots of companies now are really excited about some of these genes,” Dr. Yang noted. “They are essential for aspects of repairing DNA. There's not much we know yet about the potential efficacy and safety liability of a drug targeting these genes. We and others are actively doing research in these areas.”
Dr. Yang said that some of these genes are known to “stabilize” the CAG repeats, which tend to expand in the brain areas affected by HD. Such "somatic" repeat expansion is thought to be a key mechanism in the disease.
A gene with great potential is MSH3, a MMR gene under investigation by academic labs and biopharma firms. Before it had to shut down for lack of funding, Triplet Therapeutics had planned to use an ASO to target MSH3 in a clinical trial.
“So far, I can tell you MSH3 looks pretty safe, at least in animal models,” Dr. Yang explained.
He cautioned that scientists still need to learn more about the basic biology of the HD GWAS DNA repair genes in the brain and select the best targets and therapeutics before advancing them in clinical trials in patients.
The third leg: huntingtin protein-protein interaction
The third leg of the therapeutic stool, he said, is how the huntingtin protein interacts with other proteins.
So far, researchers have discovered at least 100 proteins that could interact with huntingtin, including in different cell types and at different ages, Dr. Yang said. The interactions occur with both the normal and mutant versions of the protein.
At least one of these proteins, HAP40, binds very closely with huntingtin. Dr. Yang described HAP40 and huntingtin as “inseparable buddies.” The Yang lab is actively working on the normal function of HAP40 in the brain and whether it could have a modifier role in HD.
As with the GWAS genes, Dr. Yang stressed that research on protein-protein interaction and its potential benefit for patients is ongoing. He added that, in the search for potential drugs, the key is finding “a protein that binds to huntingtin and is required for disease, and ideally this protein is amenable to therapeutic intervention.”
Aiming to solve one of the ‘central mysteries of HD’
The recent HDF workshop’s focus on “cell-type specific biology” in HD took up the question of why certain brain cell types (i.e., neurons in the striatum and cortex) are vulnerable to degeneration.
Dr. Yang stated that it is unclear whether research on cell-type vulnerability could become the fourth leg of the therapeutic stool. “Cell-type vulnerability could be related to” the first three legs, “especially protein-protein interaction and GWAS mismatch repair genes.”
However, this does not diminish the importance of cell-type vulnerability.
“This question of selective vulnerability is really a key feature for all neurodegenerative diseases,” Dr. Yang said. “So, for Huntington it's a striatal medium spiny neuron and some of the deep-layer cortical pyramidal neurons.” In Alzheimer’s and Parkinson’s, neuronal cell types in other brain areas are affected.
“So the big question is: why, for each disease, certain types of neurons die?” Dr. Yang asked. “If we can understand this fundamental question and elucidate its mechanism, we could use the knowledge to develop new disease-specific therapies to protect neurons from degeneration.
With the workshop, Dr. Yang said, “we think the time is right to revisit what I consider one of the central mysteries for Huntington’s disease – why certain neurons are selectively vulnerable to degeneration despite that mutant huntingtin is expressed in all the cells in the body.”
As usual, this group of HD scientists used the workshop to explore new ways to solve this mystery and develop potential therapies.
At the HDF workshop: seated, from left to right, Mahmoud Pouladi, M.Sc., Ph.D., Osama Al Dalahmah, M.D., Ph.D., Ashley Robbins, Gene Veritas (aka Kenneth P. Serbin), Sarah Hernandez, Ph.D., William Yang, M.D., Ph.D. Standing, from left to right, Xinhong Chen, Andrew Yoo, Ph.D., Anton Reiner, Ph.D., Baljit Khakh, Ph.D., Nicole Calakos, M.D., Ph.D., Ed Lein, Ph.D., Beverly Davidson, Ph.D., Nathaniel Heintz, Ph.D., Harry Orr, Ph.D., Leslie Thompson, Ph.D., Myriam Heiman, Ph.D., Shawn Davidson, Ph.D., Steven Finkbeiner, M.D., Ph.D., Roy Maimon, Ph.D. (photo by Julie Porter, HDF)
Bonding with the scientists
Following our interview and tour of the lab, I made a PowerPoint presentation to Dr. Yang and other members of the lab: “Advocating for the care and cure of Huntington’s disease: a biosocial journey.”
I spoke about my family’s struggles with HD, my advocacy, and my deepening interest in the social and scientific history of the HD movement. Afterwards, I answered questions.
Once again, I bonded with a fellow Yale graduate immersed in the fight against Huntington’s disease and scientists dedicated to a cure.
The X. William Yang Research Group after hearing Gene Veritas speak on his Huntington’s disease story. Seated (from left to right) Chris Park, Ph.D., Xiaofeng Gu, M.D., Ph.D., Dr. Yang, Gene Veritas, Nan Wang, Ph.D. Standing (from left to right) Ming Yan, MPH, Masood Akram, Ph.D., Tien Phat Huynh, M.D., Ph.D., Daniel Lee, Ph.D., Nianxin Zhong, Henry Chen, Lalini Ramanathan, Ph.D., Alexandra Shambayate, Leonardo Dionisio, Amberlene De La Rocha, Linna Deng Ferguson
Thanks to Emily Farrell, Executive Assistant, Department of History, University of San Diego, for assistance with the interview transcript.
Disclosures: the Hereditary Disease Foundation covered my travel expenses to Los Angeles. In support of the HD cause, I hold a symbolic number of Ionis shares.